Application Of Biomarkers In Clinical Drug Research
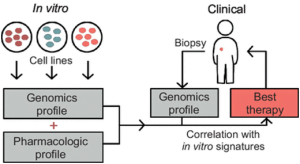
Drug discovery and development extensively drives biomedical research and primary cell culture has been a very important turning point for the drug development domain. Along with primary cell culture, a critical factor in drug discovery is the study of biomarkers. The quality of biomarker detection encourages and enhances the efficiency of drug target identification, biological drug response validation, and test subject inclusions for clinical trials. Thus, biomarkers are becoming inarguably ubiquitous in the discovery, development, and validation of novel drugs and therapies. Here are some applications that cement the effectiveness of biomarker development in the clinical drug research scenario.
1. Alzheimer’s Disease
While the clinical development of drugs against morbidity and symptoms of Alzheimer’s disease is underway, biomarkers have enabled an ease of understanding the mechanisms to effectively target tau and amyloid proteins. The USFDA have published guidelines on the role of biomarkers in prodromal Alzheimer’s disease and a research framework has also been established to increase the biomarker-based diagnostic efficiency of therapeutics based on amyloid, tau, and neurodegenerative molecules introduced by the National Institute on Aging (NIA) and the Alzheimer’s Association. The common biomarkers are: amyloid and tau, fluorodeoxyglucose-PET, and amyloid PET.
2. Polycystic Kidney Disease
Kidney diseases present various toxicities and researchers have been thoroughly studying their mechanisms and clinical characteristics for some time now. These studies mainly focus on baseline characteristics and disease-specific biomarkers. Some biomarkers in use are: albumin, IgG, kidney injury molecule−1 (KIM-1), N-acetyl-β-d-glucosaminidase (NAG), β2 microglobulin (β2MG), heart-type fatty acid-binding protein (HFABP), macrophage migration inhibitory factor (MIF), neutrophil gelatinase-associated lipocalin (NGAL), and monocyte chemotactic protein−1 (MCP-1). The qualitative assessment of genotypic and inflammatory biomarkers (such as macrophage migration inhibitory factor and MCP-1) can indicate the tubular damage in polycystic kidney disease and predict the patients’ eligibility for kidney replacements.
3. Personalized Cancer Therapy
In today’s market, at least 2,000 therapies are under clinical development for oncology treatments. The focus is all the more on precision or personalized medicine with discovery of novel immunological biomarkers in clinical research scenario. These immune-oncology markers are used in smaller late-stage trials to test for killer and helper T-cell immunotherapies. Recently, genetic biomarkers were utilized to assess the adverse effects following chemotherapy like cachexia syndrome and revealed that different protein secretome is applicable for every patient.
4. Cystic Fibrosis
Cystic fibrosis is an inheritable disease that affects the lungs and the pancreas. With the amendments in the USFDA Orphan Drug Act, cystic fibrosis therapeutics became a reality and brought about the discovery of various genetic, biochemical, and physiological biomarkers. Several diagnostic and imaging biomarkers have been identified. The G551D mutation is used as a predictive biomarker to evaluate drug efficacy whereas sweat-chloride concentration, nasal potential difference, and intestinal current were detected as in vivo biomarkers of cystic fibrosis. In addition, R117H, F508del CFTR, and G551D CFTR gene profiling can elucidate the dose-dependent drug bioactivity.
The emerging technologies of genomics, proteomics, and metabolomics with imaging and profiling advancements have led to groundbreaking discoveries of biomarkers in various diseases. Clinical drug research has thus seen a boom in biomarker-based drug developments with the help of efficient primary cell cultures to replace animal models.