Extracellular Vesicles
Featured Products
- Wistar Rat Whole Blood
- Wistar Rat Serum
- Wistar Rat Plasma
- Wistar Rat Liver S9
- Wistar Rat Liver Microsomes
- Wistar Rat Liver Cytosol
- Wistar NK cells
- Wistar Mononuclear cells
- Wistar Mesenchymal stem cells
- Wistar Dermal fibroblasts
- Wistar Dendritic cells
- Villous Mesenchymal Stem Cells
- Umbilical Cord Blood Derived Dendritic Cells
- Swiss Albino Mouse Liver S9
- Swiss Albino Mouse Liver Microsomes
- Swiss Albino Mouse Liver Cytosol
- Swine Skeletal Muscle Fibroblasts
- Swine Primary Bone Osteoblasts
- Swine Pancreatic Islets Cells
- Swine Lung Alveolar Cells
- Swine kidney Fibroblasts
- Swine Hepatocytes
- Swine Dermal Fibroblats
- Swine Cardiomyocytes
- Swine Cardiac Fibroblasts
- Swine Bone Marrow Mononuclear Cells
- Skin Dermal cells
- SD Rat Whole Blood
- SD Rat Serum
- SD Rat Plasma
- SD Rat Liver S9
- SD Rat Liver Microsomes
- SD Rat Liver Cytosol
- SD Rat Intestine S9
- SD Rat Intestine Cytosol
- SD Rat Intestinal Microsomes
- SD NK cells
- SD Muse cells
- SD Mononuclear cells
- SD Mesenchymal stem cells
- SD Dermal fibroblasts
- SD Dendritic cells
- Rhesus Monkey Whole Blood
- Rhesus Monkey Serum
- Rhesus Monkey Plasma
- Rat Schwann Cells Wistar
- Rat Schwann Cells SD
- Rat Schwann Cells Immuno-deficient
- Rat Pulmonary Fibroblasts Wistar
- Rat Pulmonary Fibroblasts SD
- Rat Pulmonary Fibroblasts Immuno-deficient
- Rat Lymphatic Fibroblasts Wistar
- Rat Lymphatic Fibroblasts SD
- Rat Lymphatic Fibroblasts Immuno-deficient
- Rat Hepatocytes Suspension Wistar
- Rat Hepatocytes Suspension SD
- Rat Hepatocytes Suspension Immuno-deficient
- Rat Hepatocytes Plateable-Wistar
- Rat Hepatocytes Plateable-SD
- Rat Hepatocytes Plateable-Immuno-deficient
- Rat Cardiomyocytes Wistar
- Rat Cardiomyocytes SD
- Rat Cardiomyocytes Immuno-deficient
- Rat Cardiac Fibroblasts Wistar
- Rat Cardiac Fibroblasts SD
- Rat Cardiac Fibroblasts Immuno-deficient
- Rat Brain Vascular Pericytes Wistar
- Rat Brain Vascular Pericytes SD
- Rat Brain Vascular Pericytes Immuno-deficient
- Rat Bone Marrow Derived NK Cells Wistar
- Rat Bone Marrow Derived NK Cells Immuno-deficient
- Rat Bone Marrow Derived Muse Cells Wistar
- Rat Bone Marrow Derived Muse Cells SD
- Rat Bone Marrow Derived Muse Cells
- Rat Bone Marrow Derived Mononuclear Cells Wistar
- Rat Bone Marrow Derived Mononuclear Cells Immuno-deficient
- Rat Bone Marrow Derived Mononuclear Cells
- Rat Bone Marrow Derived Mesenchymal Stem Cells Wistar
- Rat Bone Marrow Derived Mesenchymal Stem Cells SD
- Rat Bone Marrow Derived Mesenchymal Stem Cells Immuno Deficient
- Rat Bone Marrow Derived Dendritic Cells Wistar
- Rat Bone Marrow Derived Dendritic Cells SD
- Rat Bone Marrow Derived Dendritic Cells Immuno-deficient
- Primary Hepatocytes Plateable C 57
- Primary Hepatocytes in Suspension CD-1
- Peripheral Blood-Derived Muse Cells
- Pancreatic islets beta cells
- Muse Cells
- Mouse Primary Bone Marrow Derived NK Cells CD1
- Mouse Primary Bone Marrow Derived NK Cells C57
- Mouse Muse cells CD1
- Mouse Muse cells C57
- Mouse Muse cells BalbC
- Mouse Hybrid Liver S9 Fraction Mixed Gender
- Mouse Derived Mesenchymal Stem Cells
- Mouse Derived Dendritic Cells
- Mouse DBA S9 Fraction Mixed Gender
- Mouse DBA Lung S9 Fraction Mixed Gender
- Mouse DBA Liver S9 Fraction Mixed Gender
- Mouse Cytosol Mixed Gender
- Mouse Cardiomyocytes C57
- Mouse Cardiomyocytes BalbC
- Mouse Cardiac Fibroblasts C57
- Mouse Cardiac Fibroblasts BalbC
- Mouse C57 BL/6N Liver S9 Fraction Mixed Gender
- Mouse Brain Vascular Pericytes
- Mesenchymal Stem Cells
- Macaque Monkey blood mononuclear cells
- Lung alveolar cells
- Liver Hepatocytes plateable
- Lewis Rat Whole Blood
- Lewis Rat Serum
- Lewis Rat Plasma
- Kidney Fibroblasts
- Human Whole Blood
- Human Vaginal epithelial cells
- Human Umbilical Cord Blood Derived NK cells
- Human Umbilical Cord Blood Derived Mononuclear cells
- Human Umbilical Cord Blood Derived CD34+ Cells
- Human T Helper Cells
- Human Splenic Fibroblasts
- Human Splenic Endothelial Cells
- Human Skin S9 Fraction Mixed Gender
- Human Skin Derived Microvascular Dermal Endothelial Cells Adult
- Human Skin Derived Epidermal Melanocytes Fetal
- Human Skin Derived Epidermal Melanocytes Adult
- Human Skin Derived Epidermal Keratinocytes Neonatal
- Human Skin Derived Epidermal Keratinocytes Fetal
- Human Skin Derived Epidermal Keratinocytes Adult
- Human Skin Derived Dermal Fibroblasts Fetal
- Human Skin Derived Dermal fibroblasts Adult
- Human Skin Derived Dermal Fibroblasts Adult
- Human Seminal vesicles microvascular endothelial cells
- Human Seminal Vesicles Fibroblasts
- Human Seminal Vesicles Endothelial cells
- Human S9 Fraction Heart
- Human Pulmonary Small Airway Epithelial Cells
- Human Pulmonary Fibroblasts
- Human Pleatable Hepatocytes Pooled
- Human Plateable hepatocytes
- Human Peripheral Blood-Derived NK Cells
- Human Peripheral Blood-Derived Mononuclear Cells
- Human Peripheral Blood-Derived Monocytes
- Human Peripheral Blood-Derived Mesenchymal Stem Cells
- Human Peripheral Blood-Derived Cytotoxic T-Cells
- Human Peripheral Blood Derived Serum
- Human Peripheral Blood Derived Plasma
- Human Pericardial Fibroblasts
- Human Ovarian Surface Epithelial Cells
- Human Ovarian Fibroblasts
- Human Muse cells
- Human Microvascular Endothelial Cells
- Human Mast cells
- Human Mammary Smooth Muscle Cells
- Human Mammary Fibroblasts
- Human Mammary epithelial cells
- Human Lung S9
- Human Lung Microsomes
- Human Lung Cytosol
- Human Liver S9
- Human Liver Microsomes
- Human Liver Cytosol
- Human Kidney Fibroblasts
- Human Islets Beta cells
- Human Islet Beta Cells
- Human Intestine S9
- Human Intestine Microsomes
- Human Intestine Cytosol
- Human Hepatocytes, Plateable
- Human Hepatocytes in Suspension
- Human Eye Derived Primary Retinocytes
- Human Eye Derived Limbal Fibroblasts
- Human Extra Embryonic Fetal Tissues Muse cells
- Human Extra Embryonic Fetal Tissues Derived CD34 Positive Cells
- Human Extra Embryonic Fetal Tissues Dendritic Cells
- Human Endometrial Epithelial Cells
- Human Cytotoxic T Cells
- Human Cord Blood Derived Serum
- Human cord blood derived Plasma
- Human Cardiomyocytes
- Human Cardiac Fibroblasts
- Human Bronchial Fibroblasts
- Human Bone Marrow-Derived NK Cells
- Human Bone Marrow-Derived Mononuclear cells
- Human Bone Marrow-Derived Mesenchymal Stem Cells
- Human Bone Marrow-Derived Dendritic cells
- Human Bone Marrow-Derived CD 34 positive cells
- Human Bone Marrow Blood Derived Serum
- Human bone marrow blood derived Plasma
- Human Aortic Smooth Muscle Cells
- Human Aortic Endothelial Cells
- Human Adipose Tissue-Derived Stromal Vascular Fraction
- Human Adipose Tissue-Derived Preadipocytes
- Human Adipose Tissue derived Mesenchymal Stem cells
- Horse peripheral blood mononuclear cells
- Horse mesenchymal stem cells-adipose tissue
- Hepatic Stellate Cells
- Golden Syrian Hamster Serum
- Golden Syrian Hamster Plasma
- Gingival Fibroblasts
- Endothelial cells
- Dog mesenchymal stem cells adipose tissue
- Dog hepatocytes plateable
- Dog blood mononuclear cells
- Dental Pulp Mesenchymal Stem Cells
- Dendritic cells
- Cynomolgus Monkey Serum
- Cynomolgus Monkey Plasma
- Cynomolgus Monkey blood mononuclear cells
- Cynomolgus cryopreserved hepatocytes, plateable
- CD-1 Schwann cells
- CD-1 Pulmonary fibroblasts
- CD-1 NK cells
- CD-1 Muse cells
- CD-1 Mouse Whole Blood
- CD-1 Mouse Serum
- CD-1 Mouse Plasma
- CD-1 Mouse Lung S9
- CD-1 Mouse Lung Microsomes
- CD-1 Mouse Lung Cytosol
- CD-1 Mouse Liver S9
- CD-1 Mouse Liver Microsomes
- CD-1 Mouse Liver Cytosol
- CD-1 Mouse Intestine S9
- CD-1 Mouse Intestine Microsomes
- CD-1 Mouse Intestine Cytosol
- CD-1 Mononuclear cells
- CD-1 Mesenchymal stem cells
- CD-1 Hepatocytes plateable
- CD-1 Dermal Fibroblast
- CD-1 Dendritic cells
- CD-1 Cardiomyocytes
- CD-1 Cardiac fibroblasts
- CD-1 Brain vascular pericytes
- Cardiomyocytes
- Cardiac fibroblasts
- C57 Schwann cells
- C57 Pulmonary fibroblasts
- C57 NK cells
- C57 Muse cells
- C57 Mouse Whole Blood
- C57 Mouse Skin S9
- C57 Mouse Skin Microsomes
- C57 Mouse Skin Cytosol
- C57 Mouse Serum
- C57 Mouse Plasma
- C57 Mouse Lung S9
- C57 Mouse Lung Microsomes
- C57 Mouse Lung Cytosol
- C57 Mouse Liver S9
- C57 Mouse Liver Microsomes
- C57 Mouse Liver Cytosol
- C57 Mouse Intestine S9
- C57 Mouse Intestine Microsomes
- C57 Mouse Intestine Cytosol
- C57 Mouse Heart S9
- C57 Mouse Heart Microsomes
- C57 Mouse Heart Cytosol
- C57 Mononuclear cells
- C57 Mesenchymal stem cells
- C57 Hepatocytes Suspension
- C57 Dendritic cells
- C57 Cardiomyocytes
- C57 Cardiac fibroblasts
- C57 Brain vascular pericytes
- Brown Norway Rat Whole Blood
- Brown Norway Rat Serum
- Brown Norway Rat Plasma
- Beagle Whole Blood
- Beagle Serum
- Beagle Plasma
- Beagle Dog hepatocytes cryopreserved, plateable
- BalbC Schwann cells
- BalbC Pulmonary fibroblasts
- BalbC NK cells
- BalbC Muse cells
- BALBC Mouse Whole Blood
- BALBC Mouse Serum
- BALBC Mouse Plasma
- BalbC Mononuclear cells
- BalbC Mesenchymal stem cells
- BalbC Hepatocytes Suspension
- BalbC Hepatocytes plateable
- BalbC Dermal Fibroblasts
- BalbC Dendritic cells
- BalbC Cardiomyocytes
- BalbC Cardiac fibroblasts
- BalbC Brain vascular pericytes
- BALB/c Mouse Skin S9
- BALB/c Mouse Skin Microsomes
- BALB/c Mouse Skin Cytosol
- BALB/c Mouse Lung Cytosol
- BALB/C Mouse Liver S9
- BALB/c Mouse Liver Microsomes
- BALB/c Mouse Liver Cytosol
- BALB/c Mouse Intestine S9
- BALB/c Mouse Intestine Microsomes
- BALB/c Mouse Intestine Cytosol
- BALB/c Mouse Heart S9
- BALB/c Mouse Heart Microsomes
- BALB/c Mouse Heart Cytosol
- Amniotic Epithelial cells
Drop your Query
Recently, extracellular vesicles have attracted considerable interest from the scientific community due to their multiple applications including therapeutics; drug delivery, and biomarkers. Considering that in today’s era of personalized medicine, wherein targeted delivery of drugs is crucial for effective therapeutic outcomes; the role of exosomes in drug delivery has been a recent topic of interest in the field of nanotechnology.
Special interests were raised, when researchers all over the world understood that extracellular communication help in transmitting messages through intracellular communication. Traditionally, it was well known that cells secrete certain vesicles during their growth period, but previously it was thought that during cellular communication, signals were sent to promote apoptosis in the form of extracellular vesicles. However, with the recent investigation, it is well-known that healthy cells also secrete extracellular vesicles to promote a healthy growth environment in the tissue.
| Exosomes | Micro vesicles | Apoptotic Bodies | |
| Origin | Endocytic Pathways | Plasma Membrane | Plasma Membrane |
| Size | 40-120 nm | 50-1,000 nm | 500-2,000 nm |
| Function | Intercellular Communication | Intercellular Communication | Facilitate Phagocytosis |
| Markers | CD 81, CD 63, CD 9 | Integrin, selectin | Annexin V |
| Contents | Proteins and nucleic acids | Proteins and nucleic acids | Nuclear fractions, cell organelles |
Exosomes and microvesicles (MVs) are released by both healthy and diseased cells. Although, it should be noted that exosomes secreted by both cell types differ in several factors. While in the neurogenic system, EVs have been proposed to participate in cell-to-cell transit systems by mediating short- and long-distance communication, affecting various aspects of cell biology.
Considering their functional importance, currently, exosomes are being explored for their therapeutic benefits, as potential biomarkers, and as an effective drug delivery system.
Kosheeka has come up with a range of therapeutic exosomes and oncogenic exosomes, derived from good quality, growing primary cells and cancer cells.
With recent advancements in the diagnostic industry, biomarkers are being referred to as the new innovative signatures to understand disease pathophysiology; further supporting drug screening and drug discovery. Given the fact that exosomes play a critical role in cellular communications while being loaded with important information in the form of proteins, lipids, and messengers; the same can be referred to as diagnostic checkpoints of abnormalities at the cellular levels when compared with healthy control. Thus, exosome-mediated detection technologies are emerging currently in the field of diagnostics, as the early detection of disease status may lead to efficient treatment for various chronic diseases including cancer, autoimmune disorders, and inflammatory diseases. Accordingly, many studies have confirmed the relevant importance of diagnostics. The details of the same can be obtained herewith:
- A study confirmed that circulating Exo -DNA with higher mutational probability in KRAS genes can be used as a confirmatory test for the entry-stage development of pancreatic cancer (Allenson K., et.al; 2017).
- Another study confirmed increasing levels of GPC1+ circulating exosomes to be referred to as an indication of pancreatic ductal carcinoma and colorectal cancers (Melo, et.al; 2015).
- In lung cancers, the detection of circulating exosomes EGFRT790M has great potential as a confirmatory test, further avoiding unnecessary tumor biopsies (Castellanos, et.al; 2018)
- A study also confirmed that the expression of proteins can potentially discriminate between cancerous and non-cancerous growth. A study has revealed that the expression of CD151, CD171, and tetraspanin 8 can be considered the most significant to separate patients with lung cancer and cancer-free individuals (Sandfeld-Paulsen, et.al; 2016).
- In lung cancer the FLI1 exonic circular RNAs were identified as the novel carcinogenic driver, further contributing to the metastasis of cancer; can be used as an effective biomarker confirming cancer metastasis (Li L, et.al; 2019).
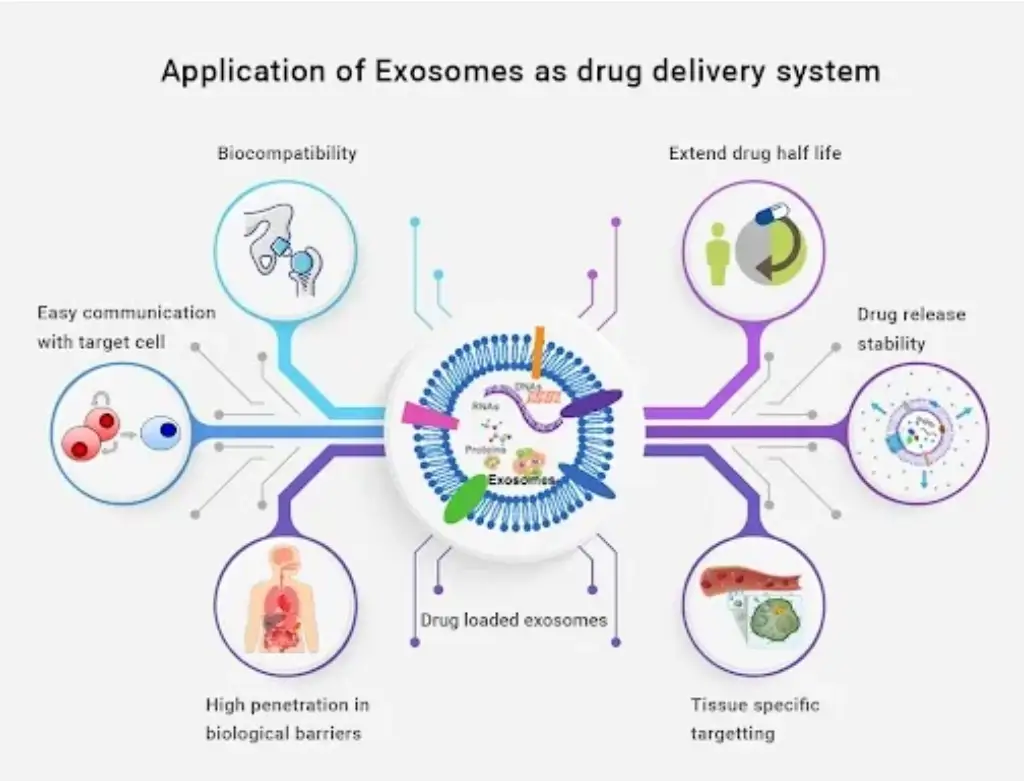
EVs possess inherent properties of tissue repair and overall remodeling, which can further be exploited for therapeutic benefits. In the context of regenerative medicine and cell-based therapies, EVs show significant benefits in comparison to their source, EVs may be less immunogenic.
Unlike cellular components, EVs have a longer shelf life and can be circulated in the blood system for approximately 3 months. EVs do not replicate after injection and can cross the blood-brain barrier very easily. Thus, since the time of their discoveries, EVs have served as potential therapeutic agents in the liver, neurodegenerative as well as cardiovascular disorders. Exosomes often inherit properties of their parent cells, and hence for therapeutic benefits stem cell-derived exosomes are identified to be potential candidates to redefine the future of medicine.
Many preclinical animal models have successfully confirmed the therapeutic potential of EVs derived from mesenchymal stem cells. A few examples of studies include:
- At the preclinical setup, it has been confirmed that progressive liver fibrosis can be ameliorated with the infusion of UCMSC-derived exosomes that are 30-150 nm in the range (Li et.al; 2013)
- Another study confirmed through preclinical mouse models that hUCMSC-derived exosomes can ameliorate experimental autoimmune uveoretinitis through the inhibition of inflammatory cellular migration (Bai et.al; 2017)
- EVs from hUCMSCs are also known to inherit therapeutic potential in multiple neurodegenerative ailments including Alzheimer’s disease, Huntington’s Disease, etc. (Cho et.al; 2018)
Few published studies have demonstrated the effectiveness of exosomes in clinical setups.
- Currently, hUCMSC-EVs are being explored as potential cell-free therapeutic options with characteristic protective and immunomodulatory properties (Gowen et.al; 2020)
- Another study further highlighted the broad-spectrum therapeutic properties of MSC-derived EVs, introducing their ability to reduce damage associated with chronic kidney injuries; a disease characteristically featured by its strong fibrotic, inflammatory, and apoptotic components (Ramirez-Bajo et.al; 2020).

Over the past couple of years, multiple research projects have been ongoing to improve methods for targeted drug delivery; especially in the case of cancer. Out of various methods that have been investigated, a targeted exosome-based drug delivery system has been proven to be highly effective. Their natural designation as cargo transporters lends credence to the belief that exosomes are naturally occurring extracellular particles; specifically involved in the targeted delivery of various biological materials. Researchers can hijack this inherent property of exosomes for effective therapeutic applications, including drug delivery at the site of injury.
Other than exosomes, other nanoparticles like liposomes, as well as polymeric NPs are also used as targeted delivery systems with good stability as well as long-circulating capabilities. However, using these nanoparticles also has regulatory obligations; hence, exosomes can be the most preferred choice.
A few studies that have shown the exosome’s ability to deliver drugs are as follows:
- A study was conducted to analyze the drug delivery property of exosomes, wherein MSC-derived exosomes were complexed with curcumin. The therapeutic outcome of the exosome-curcumin complex was compared with that of free curcumin in mice models. The study further confirmed that the exosomes-curcumin complex enhanced clinical efficiency (Aggarwal et.al; 2009).
- Another in vitro experimental model confirmed that complexes of drugs formed with exosomes can significantly reduce inflammatory cytokine levels than native drugs only (Dhillon et.al; 2008)

- Aggarwal, B.B.; Harikumar, K.B. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune, and neoplastic diseases. Int. J. Biochem. Cell Biol. 2009, 41, 40–59.
- Allenson, K. et al. High prevalence of mutant KRAS in circulating exosome-derived DNA from early-stage pancreatic cancer patients. Ann. Oncol.28, 741–747 (2017).
- Dhillon, N.; Aggarwal, B.B.; Newman, R.A.; Wolff, R.A.; Kunnumakkara, A.B.; Abbruzzese, J.L.; Ng, C.S.; Badmaev, V.; Kurzrock, R. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin. Cancer Res. 2008, 14, 4491–4499
- Melo, S. A. et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature523, 177–182 (2015).
- Li, J. et al. GPC1 exosome and its regulatory miRNAs are specific markers for the detection and target therapy of colorectal cancer. J. Cell. Mol. Med.21, 838–847 (2017).
- Castellanos-Rizaldos, E. et al. Exosome-based detection of EGFR T790M in plasma from non–small cell lung cancer patients. Clin. Cancer Res.24, 2944–2950 (2018).
- Sandfeld-Paulsen, B. et al. Exosomal proteins as diagnostic biomarkers in lung cancer. J. Thorac. Oncol.11, 1701–1710 (2016).
- Li, L. et al. FLI1 exonic circular RNAs as a novel oncogenic driver to promote tumor metastasis in small cell lung cancer. Clin. Cancer Res.25, 1302–1317 (2019).
- Li, T., Yan, Y., Wang, B., Qian, H., Zhang, X., Shen, L., et al. (2013). Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev. 22, 845–854. DOI: 10.1089/scd.2012.0395
